

.jpg)
2019
September 5,2019, Shanghai, China - HaiHe Biopharma, a biopharmaceutical company focusing on the discovery, development and commercialization of innovative anti-tumor drugs, and Daehwa Pharmaceutical Co., Ltd (hereinafter referred to as "Daehwa Pharmaceutical") jointly announced that the first subject has been dosed in the Phase III clinical trial of oral paclitaxel RMX3001 jointly developed by the two companies in the treatment of breast cancer in China.
This is an international, multicenter, open-label, phase III clinical trial to evaluate the efficacy and safety of Liporaxel ®(oral paclitaxel ) vs. Taxol ®(Taxol ®) as the first-line treatment in patients with recurrent or metastatic HER2 negative breast cancer. The principal investigator of this clinical trial is Professor Xu Binghe from Cancer Hospital, Chinese Academy of Medical Sciences.
According to 2012 global cancer statistics, breast cancer is the most common malignant tumor in women, with about 1.7 million new cases and about 500,000 deaths each year. In 2015, there were more than 272,000 new cases of breast cancer and about 70, 000 deaths in China, and the incidence and mortality of breast cancer have increased significantly in recent years. The overall median survival time for patients with advanced breast cancer after diagnosis is 2-3 years, and the 5-year survival rate is only about 20%. Once a distant organ metastasis occurs, the possibility of cure is very limited, or even does not exist.
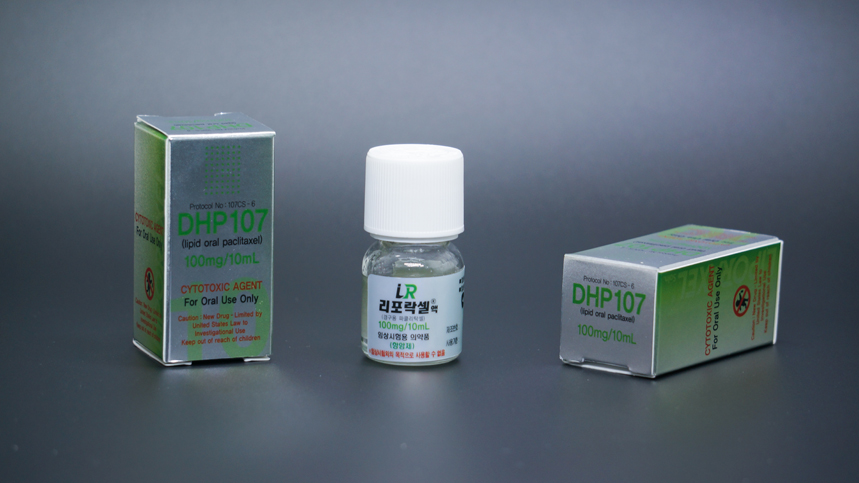
Paclitaxel is one of the most extensively used chemotherapy drugs with huge market demands. Currently, the marketed dosage form of the drug is injection, which is inconvenient to use and has many side effects. Therefore, the development of oral paclitaxel dosage forms has been a research focus in the pharmaceutical industry. With the trade name of Liporaxel®, this product is an oral paclitaxel dosage form developed by DAE HWA Pharmaceutical based on its innovative technology of lipid self-emulsifying drug delivery, which has been approved for the indication of gastric cancer by the South Korean Ministry of Food and Drug Safety (MFDS) on September 9, 2016. To date, Liporaxel® is the first oral paclitaxel product that has been successful developed and approved in the world. In September 2017, HaiHe Biopharma obtained the rights of the product in mainland China, Taiwan, Hong Kong, and Thailand from DAE HWA Pharmaceutical.
Haihe Biopharma is an innovation-driven biotechnology company in China focusing on the discovery, development, production and commercialization of innovative anti-tumor drugs. Haihe brings life-saving therapies to cancer patients worldwide. It also has a research and management team with a global perspective, and is proactively mapping out the international development of innovative drugs. The Company currently has thirteen key drug candidates. As of today, Haihe Biopharma has received 21 IND or clinical trial approvals in four countries and regions.
Please visit the company website for more information: http://www.zztsbc.com
DAEHWA Pharmaceutical Co., Ltd., was established in the spirit of “providing society a more human-centered pharmaceutical business.” Its main goal is to improve National health and people’s life. Since its foundation in 1984, DAEHWA continuously seeks new paths for its business development with a consistent progress-oriented core-target. With its own platform technologies, DAEHWA produces APIs and finished products. Liporaxel Solution, which is the result of DAEHWA’s 17-year study. The completed clinical studies proved its efficacy, safety and convenience. DAEHWA invests more than 10% of its sales into R&D, is a global company specialized in pharmaceutical products.
Please visit the company website for more information: http://www.dhpharm.co.kr